| |
FROM NORMAL BRAIN AGING TO DISEASES AFFECTING THE NERVOUS SYSTEM
|
Brain aging is the major risk factor for developing dementia, neurodegenerative diseases and cancer, and it is associated with a decreased buffering capacity of the proteostasis machinery. One of the main components of the proteostasis network altered during aging involves the function of the ER. To cope with ER stress, the UPR reprograms gene expression by the concerted action of specialized transcription highlighting ATF6, XBP1 and ATF4. Our laboratory has been central in defining the significance of the UPR to neurodegenerative diseases, normal brain function and more recently brain aging. We have expanded our studies to brain cancer and psychological stress.
Neurodegenerative diseases
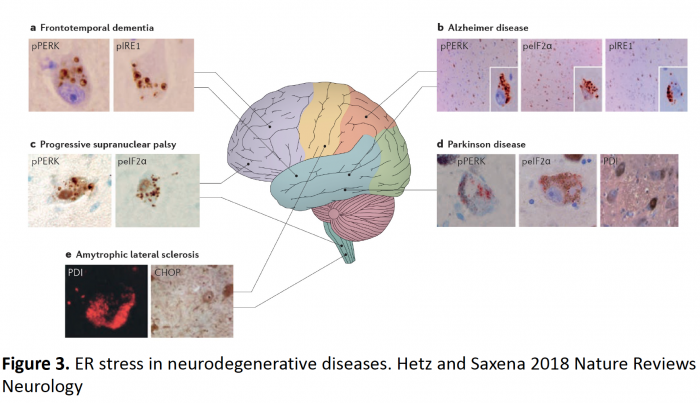
Most neurological diseases are linked with the abnormal protein aggregation. These diseases also share the induction of ER stress (Figure 3). For almost 2 decades, we have investigated the role of ER stress and the UPR to different diseases using genetic manipulation of distinct components of the pathway, including amyotrophic lateral sclerosis (ALS), Huntington’s disease, Parkinson’s disease, Alzheimer’s disease and Prion-related disorders. Our studies revealed a highly complex scenario where distinct UPR components could have contrasting and even opposite effects depending on the disease analyzed (context dependent).
Synaptic function
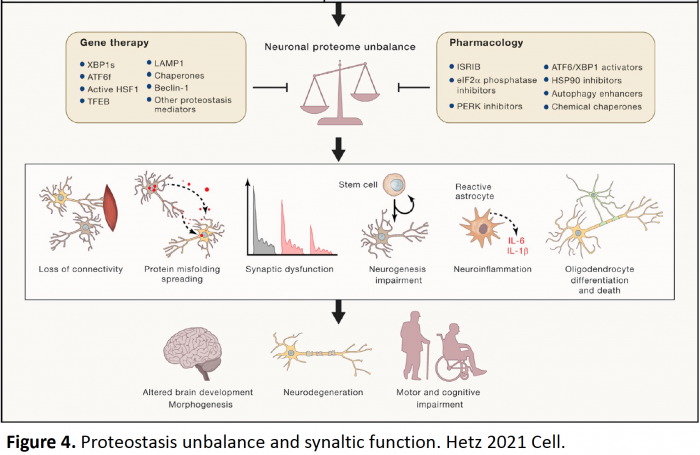
Neuronal proteostasis is maintained by the dynamic integration of different processes that regulate the synthesis, folding, quality control, and localization of proteins. The UPR plays a central role in the quality control of many ion channels and receptors, in addition to crosstalk with signaling pathways that regulate connectivity, synapse formation, and neuronal plasticity. We have contributed to understand the role of the UPR to brain development, neuronal physiology, and behavior. We reported that XBP1 is central to regulate synaptic plasticity, impacting learning and memory performance possibly due to the regulation of BDNF expression (Martinez et al., 2016 Cell Rep). In addition, in the context of brain aging, IRE1 and XBP1 contribute to sustain normal brain aging. Artificial enforcement of XBP1 in the hippocampus restored near 70% of the proteomic changes associated with normal aging, impacting the production of proteins involved in synaptic activity, connectivity, and neuronal function (Cabral et al., 2021 EMBO J). We prose that perturbations in the proteostasis network results in serious unbalance of the neuronal proteome, driving the emergence of cognitive and behavioral problems (Figure 4) (Hetz 2021 Cell).
Brain cancer
Glioblastoma multiforme (GBM) is the most common malignant brain tumor. Despite tumor resection followed with radiation and chemotherapy, the patient’s overall survival is only 15 months. The UPR contributes to cancer by promoting tumor growth and survival, and oncogenic transformation. Recent evidence suggest that cancer cells propagate ER stress signals from tumors to surrounding stromal cells through a mechanism known as “transmissible ER stress (TERS)”, reprogramming stroma. We are currently developing a systematic study in complementary model systems to manipulate the UPR in GBM stroma with state-of-the-art genetic and pharmacological approaches, followed by molecular, cellular, and functional characterization. This strategy will enable us to define the functional significance of TERS to GBM progression.
Psychosocial stress
COVID-19 pandemic had devastating consequences to mental health, especially on the elderly. The negative effects on brain function of the exposure of individuals to chronic stress involve complex intracellular signaling pathways and tissue crosstalk. Subcellular organelles in neurons, including the endoplasmic reticulum (ER) are aletered in different psychiatric disorders and a polymorphism on the XBP1 promoter is linked to bipolar disorders and schizophrenia in Japan (Diaz and Hetz 2021 Trends Endo Met). We are investigating the significance of ER proteostasis to stress-induced behavioral alterations using mouse models and multimodal stress (mCRS) paradigm.
Intellectual disability and ER protein folding
Recessive gene mutations underlie many developmental disorders and often lead to disabling neurological problems. Previously, we identified rare variants on protein disulfide isomerases (PDIA) in ALS patients, which impacted neuromuscular junction stability by altering the production of important synaptic proteins (Woelbier et al., 2016 EMBO J. Rozas et al., 2021 Acta Neuropathol Com). PDIs are central ER foldases catalyzing the formation of disulfide bonds in proteins. We recently identified of a homozygous c.170G>A (p.Cys57Tyr or C57Y) mutation in the gene coding for protein disulfide isomerase A3 (PDIA3, also known as ERp57) in patients with syndromic intellectual disability. PDIA3C57Y expression impairs synaptic plasticity and memory consolidation, involving dysregulation of cell adhesion and actin cytoskeleton dynamics (Medinas et al., 2021 EMBO J). We are currently investigating the mechanisms relating PDI with synaptic function, and developing new mouse models of intellectual disability.